mercury valence electrons|how many electrons in mercury : Cebu Find the valence electrons of mercury and other elements in a chart with atomic number and elements. Learn how to determine the valence electrons of main group, transition . Juta88.com is a trusted Malaysia Online Casino. Juta88 offer a wide selection of slot games, Live Casino, Sports Betting, Mega888, Bonus888 and many more with 100% welcome bonus.
PH0 · periodic table number of valence electrons
PH1 · of valence electrons in hydrogen
PH2 · mercury noble gas configuration
PH3 · mercury electron configuration
PH4 · how many electrons in mercury
PH5 · hg valence electrons
PH6 · br valence electrons
PH7 · Iba pa
Project Gutenberg is an online library of free eBooks. Project Gutenberg was the first provider of free electronic books, or eBooks. Michael Hart, founder of Project Gutenberg, invented eBooks in 1971 and his memory continues to inspire the creation of eBooks and related content today.
mercury valence electrons*******Find the valences of all elements in the periodic table, including mercury, which has valences of +1 and +2. Learn how valences are determined by electron configurations and shell filling.Find the valence electrons of mercury and other elements in a chart with atomic number and elements. Learn how to determine the valence electrons of main group, transition .Learn how to write the complete electron configuration of mercury (Hg) using Bohr's atomic model and Aufbau principle. See the electron arrangement in different orbitals and .
This means that the chemistry of an atom depends mostly on the electrons in its outermost shell, which are called the valence electrons. The simplified notation allows .
The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into .how many electrons in mercuryMercury is a liquid metal with two valence electrons and an atomic number of 80. It is a toxic and rare element that can cause neurological damage in children and adults.Electron configuration for mercury. The history of Mercury. Periodic table history. Identifiers. List of unique identifiers for Mercury in various chemical registry databases. Mercury is a .Recent News. Mar. 14, 2024, 6:51 AM ET (The Hindu) Mercury in political barometer shoots up ahead of peak summer. mercury (Hg), chemical element, liquid metal of Group 12 .mercury valence electrons how many electrons in mercuryValence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number .About. Transcript. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = . Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .Mercury is a chemical element of the periodic table with chemical symbol Hg and atomic number 80 with an atomic weight of 200.592 u and is classed as a transition metal. . Valence electrons : 2: Valency electrons : 1,2: Bohr model: Electron shell for Mercury, created by Injosoft AB Hg. Figure: Shell diagram of Mercury (Hg) atom. Orbital .Now, the electron configuration of mercury shows that the last shell of mercury has two electrons and the 5d orbital has a total of ten electrons. Therefore, the valence electrons of mercury are two. The last electron of mercury enters the d-orbital. Therefore, it’s a d-block element. To know these properties of mercury one must know the .mercury valence electrons So, we just added the individual valence electrons of each atom separately. The electronic configuration of Hg is [Xe]4f 14 5d 10 62 2; So, the valence electrons present over the Hg atom is 2, as 6s is the valence orbital of Hg; So, the total number of valence electrons for the Mercury is 2+2 = 4; 3. Mercury structure lone pairs. The lone .
How many valence electrons are in the ground state electron configuration of mercury? Given: atomic number. Asked for: complete electron . For chemical purposes, the most important electrons are those in the outermost principal shell, the valence electrons. Problems. Unless specified, use any method to solve the following problems. Answers . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for main group elements. For the transition metals with partially-filed d shells, valence electrons are those electrons outside the noble gas core.Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a .
The element Mercury was discovered by Indians/Chinese in year Before 2000 BCE . Mercury was first isolated by Egypt in 1500 BCE. How many valence electrons does a Mercury atom have? Mercury has 2 .
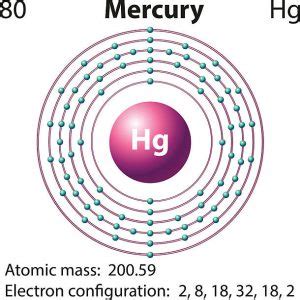
Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.

Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.
Mercury has two valence electrons, both of which sit in the atom’s 6s shell. A valence electron is an electron in the outermost shell of an atom. Valence electrons are capable of bonding with the valence electrons of other atoms to form a compound. The electron configuration of mercury can be used to determine the number of valence . What makes mercury special is its electron configuration: [Kr] 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2. The filled 4f shell poorly shields valence electrons from the positive nuclear charge. The 6s electrons draw close to the atomic nucleus, shrinking the atomic radius. Orbiting such a large nucleus means the electrons move at relativistic speeds . To find the number of valence electrons for Gold (Au) we need to look at its electron configuration. This is necessary because Au is a transition metal (d b.The atomic number of mercury is 80, which means it has 80 electrons. Now it is possible to find the orbital notation of mercury very easily through electron configuration. That is, the orbital notation of mercury is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10.We would like to show you a description here but the site won’t allow us.
Current local time in Japan – Tokyo. Get Tokyo's weather and area codes, time zone and DST. Explore Tokyo's sunrise and sunset, moonrise and moonset.
mercury valence electrons|how many electrons in mercury